| Introduction |
| Technical aspects |
| Normal lymph node histology and cytology |
| Reactive lymph node |
| Hodgkin Lymphoma |
| Non Hodgkin lymphoma |
| Metastases |
TECHNICAL ASPECTS
- FNC and smear preparation
- Basic staining
- FNC material management and ancillary techniques
- Cytospin
- Cell block
- Immunocytochemistry (ICC)
- Flow cytometry (FC)
- Fluorescence in situ hybridization (FISH)
- Polymerase chain reaction (PCR)
FNC and smear preparation
Back to top
- FNC of palpable lymph nodes should be performed using a 23- or 25-gauge needle, with or without aspiration. Small lymph nodes (1 cm or less) may be easier to approach with a shorter needle, without aspiration equipment, to get closer to the target.
- Block the lymph node between two fingers; in case of axillary lymph nodes try to immobilize the target on the thoracic wall by “pulling it down” with two fingertips.
- Try to shorten the distance between the skin and the target and drive the needle proportionally to the distance needed to pass through it.
- FNC of impalpable or deep lymph nodes needs to be performed under ultrasound control. In this case chose the shortest and more direct way: the longer and more oblique the way, the higher the risk of deviation of the needle. Needle movements are limited by the driver; if the lymph node is sufficiently large, try to reach the most significant part of it.
- Follow the needling perceptions: the skin offers the first resistance, the capsule less; getting in the lymph node, you may feel as if you had reached a cavity; if so, you have reached the target.
- Gently move the needle up, down and sideways drawing a radius proportional to the size of the target.
- Moving the needle, ask the patient if he/she feels pain: if so, you may be out of the target.
- Evaluate the needling: soft, hard, fibrous, crushing; it might help you in the evaluation of the smears.
- Check the hub of the needle during aspiration; when you begin to draw material, stop the FNC and prepare the smears. If you see blood in the hub of the needle, stop the pass immediately; blood damages your smear and micro-haemorrhage may compromises a second pass.
- Aspirations should not exceed 20-30”; apart from cases which are “fibrous” at needling, longer aspirations may be useless or cause micro-haemorrhages.
- Flush the aspirated material very gently; if you divide it into small drops you can prepare additional smears from the same pass or use the material for cell-block and ancillary techniques.
- Choose the smearing technique in reason of the quality of the material: hematologic technique for fluid material, and direct smear for dense material.
- Smearing should be light enough to obtain monolayer smears which immediately air dry, and gentle enough to preserve the morphology of the lymphocytes.
- Immediately examine a Diff Quik stained smear for adequacy and to decide if additional passes are needed and how to manage the material.
Basic staining
Diff Quik and Papanicolaou are complementary and the most utilized types of staining. Diff Quik provides an immediate evaluation of the smears, keeps the background, highlights fibrous connective tissue metachromasia and the orange granules of eosinophils. Papanicolaou provides detailed information on the nucleus and cytoplasm; if necessary, the smear can be distained and used for immunocytochemical stains.
Back to topFNC material management and ancillary techniques
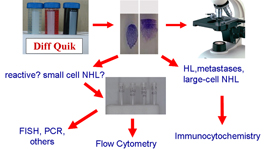
Management of diagnostic material is a crucial point in diagnostic cytology, and mainly in lymph node cytopathology; perfect smears and their immediate evaluation are the first steps of a correct and accurate diagnosis. Ancillary techniques are necessary for accurate diagnosis and indispensable in cases of non-Hodgkin lymphoma (NHL). Nonetheless not all these techniques are available in basic cytology laboratories and each of them has its own specific applications. In fact, FC, which provides extremely useful information for the diagnosis and often the classification NHL, is unsuitable to diagnose Hodgkin lymphoma (HL) and metastases. Conversely, ICC by combining morphological details and immunophenotypic features, is highly contributive in the diagnosis of HL and metastases but may be less effective in light chain evaluation or in the identification of T-rich B-cell NHL. FISH is highly effective in the identification of specific NHL entities but is generally used only when there is substantial diagnostic orientation. These problems, the general scantiness of FNC material and the need to capitalize technical resources have suggested the following algorithm.
Ancillary techniques are indispensable in the diagnosis and classification of most of the lymph node FNC. Each of them may produce specific information and should be used considering the clinical data and cytological features.
Back to topCytospin
The material left in the hub of the needle and/or obtained from a second pass may be suspended in buffered solution at pH 7.4. One or two million cells, easily obtained from one pass, are needed to prepare six or more cytospins to be used for immunocytochemistry. Air dried cytospins can be stored at room temperature for up to 1 week; for longer periods (1-2 years) they need to be stored at -20°. Both immunoalkaline phosphatase and immunoperoxidase can be used on cytospin.
Back to topCell block
Cell blocks can be prepared by embedding centrifuged cell suspensions in paraffin; Cytoblock cassettes for formalin fixed cell suspensions may be used. The main advantages of cell blocks are the numerous sections they produce, their long term storage, and the same antigenic conditions as formalin fixed histological sections.
Back to topImmunocytochemistry (ICC)
Ethanol fixed smears or xylene dewaxed and alcohol rehydrated paraffin sections obtained from cell-blocks can be used for this purpose. Slides are placed in Coplin jars filled with a 0.01 M tri-sodium citrate solution, and microwave-heated three times for 5 minutes. After heating, slides are thoroughly rinsed in cool running water for 5 minutes and then washed in Tris-Buffered Saline (TBS) pH 7.4. After incubation with the primary Ab, slides are covered with biotinylated anti-mouse or anti-rabbit immunoglobulins, followed by peroxidase labelled streptavidine (LSAB); the signal is developed using diaminobenzidine (DAB) as chromogen after incubation for 10 minutes with Horse Peroxidase Enzime (HRP).
Back to topFlow cytometry (FC)
The remaining material in the hub of the needle or from additional passes can be suspended in RMPI-1640 or in buffered solution at pH 7.4. One or two million cells, which can be collected in one pass, are distributed into six tubes for a basic panel of fluoresceinated antibodies. The latter are used in groups of three or even four antibodies conjugated with different fluorochromes using double lasers instruments. If possible, it may be useful to keep one tube for further evaluation. Fluoresceinated antibody conjugation has to be performed timely because vital cells may swell in the suspension. After conjugation, cell swelling may be prevented by fixation, adding a drop of buffered formalin; analysis may thus be postponed or repeated. Advantages of FC are: direct reaction antigen-antibody, exact quantification of antigen expression, possible co-expression of two different antibodies on the same cells. Disadvantages are the absence of morphology, loss of large cells, difficulty to identify numerically scanty cell populations.
Back to topFluorescence in situ hybridization (FISH)
Fluoresceinated DNA probes are used to visualize specific DNA segments. Chromosomal abnormalities, namely translocations and deletions may be observed and quantified. FISH may be performed on smears or cytospins. The advantage of using cytospins are: high cells concentration, probe sparing and short analysis time.
Back to topPolymerase chain reaction (PCR)
Aspirated cells may be suspended in RNAlater® for both RNA and DNA preservation and extraction. Cells may be stored at room temperature until DNA extraction. DNA segments are amplified using oligonucleotide probes and polymerase enzymes in repetitive cycles of denaturation, annealing and extension. The amplification products may be analysed by gel electrophoresis or capillary electrophoresis. The JH locus of Ig heavy chains or Tc receptor may be amplified and evaluated to assess monoclonality or polyclonality.
Back to top