| The Pap Test |
| Collection of specimens |
| Guidelines on laboratory organisation, processing and screening |
| Terminology and reporting |
Guidelines on laboratory organisation, processing and screening
Stages in the flow of work through a cytology laboratory:
- Specimen and request form received by the laboratory
- Laboratory reference number assigned
- Patient ID and smear reference number entered on lab computer
- Smears stained, mounted and labeled
- Slides screened by primary screener
- All slides checked by second screener and abnormals refered to pathologist
- Final report prepared and dispatched to smear taker
- Patient informed of result of test
- Insure appropriate action taken in event of abnormal result
Reception of specimens
- On reception in the laboratory the smear and request form should be checked to ensure they match. Both slide and request form should be given the a same laboratory reference number or barcode
- The slide should be prepared for staining by the Papanicolaou method
- After staining the slide should be mounted in DPX and covered with a coverslip (optimal size 22x50mm).
- The mountant should be allowed to dry before the slide is matched again with the corresponding request form and sent for screening
The Papanicolaou staining method
After many years of experimentation, Dr Papanicolaou developed the trichrome stain which has survived with little modification to this day and is the accepted stain for cytological preparations ( Papanicolaou 1942: A new procedure for staining vaginal smears Science 95,438). For optimal staining the smears must not be allowed to dry at any time before fixation or during processing
Papanicolaou stain
Procedure Time Comment Remove polyethylene glycol carbowax fixative In 50% alcohol 2 min's Failure to remove carbowax will result in artifact which obscure the cells & is difficult to remove Rinse in water
1 min's Ensure re hydration is complete Stain in Harris Haematoxylin 5 min's Staining time will depend on formula of haematoxylin Rinse in water 2 min's Unbound haematoxylin is removed Differentiate in 0.5% aqueous hydrochloric acid 10-20 sec's Rinse in tap water 2 min's Blue in Scott’s tap water substitute 1 min's The brown colour of acid haematin is changed to blue/ black by weak alkaline solution Rinse in water 2 min's Wash thoroughly to remove alkaline salts Dehydrate 70%alcohol 2 min's Dehydrate through graded alcohols Dehydrate 95%alcohol 2 min's Dehydrate 95%alcohol 2 min's Stain in Orange G6 (OG6) 2 min's Rinse in 95%alcohol 2 min's Rinse in 95% alcohol 2 min's Rinse in 95% alcohol 2 min's Stain in Gills EA30 solution 3 min's Rinse in 95% alcohol x3 1 min Clear in xylene x3 and mount in DPX
1 min Leave in xylene until ready to mount in DPX Resulting stain:
- Nuclei- blue black
- Cytoplasm- non keratinised blue and effete red
- blood cells- green
- Cytoplasm (keratinised)- pink
- Red cells- orange
The end result should retain the transparent quality of the cytoplasm and the chromatin structure of the nuclei should be clearly defined.
Comment on the Papanicolaou staining method:
The Papanicolaou stain is a polychrome staining method which comprises a nuclear stain (haematoxylin) and two counterstains (Orange G and EA dyes). Hydration of the fixed smear is required for the cells to take up the haematoxylin whereas dehydration prepares the smear for the counterstains. Modification of the stain is common as each laboratory prefers its own colour balance. Adjustments are made by altering the length of time in haematoxylin and EA dye. Other factors which may affect the colour balance are chemical content of the tap water, temperature, pH of specimen and number of slides per batch of stain. Providing that the nuclear detail is clearly defined and the transparency of the cytoplasm is maintained, interlaboratory variation of the colour balance is acceptable. However each laboratory should standardise its procedure so the results are reproducible.The Papanicolaou staining method may progressive or regressive.
In the progressive method the intensity of nuclear staining is controlled by immersion of the slide in a “blueing” agent after the nucleus has been stained to the required intensity with haematoxylin. The blueing agents most commonly used are Scott’s tap water substitute (pH 8.02), ammonium hydroxide and lithium carbonate. (Tap water can be used if the pH is suitable). Progressive staining tints the cytoplasm very lightly.
In regressive staining, the nucleus is deliberately overstained with a non-acidified
haematoxylin. The excess stain is removed with dilute hydrochloric acid solution .The decolourising acid is then removed by immersing the slide in running tap water .Timing is important in the regressive method as the outcome may be a hypochromatic nucleus rather than a hyperchromatic nucleus. The cytoplasm is also totally decolourised by the acid solution. Harris’s haematoxylin is usually combined with the regressive staining method .Gill’s or Mayer’s haematoxylin is usually used for the progressive staining method. It is important to remember that the pH of the solution is more important than whether the haematoxolin is used progressively or regressively.Orange G is an acidic dye which stains basic proteins such a prekeratin a pink colour. Orange G also has a strong affinity for keratin which stains bright orange. Thus it is an important marker of abnormal keratinisation of the cervical and vaginal epithelium such as occurs in prolapse and wart virus infection. It is also a sensitive marker of well differentiated squamous carcinoma of the cervix. It is of utmost importnace that the rinsing baths are clean and the staining solutions are changed frequently otherwise both nuclear and cytoplasmic staining will be defective.
EA is a polychromatic stain which is a combination of light green, SF yellow and eosin Y. It stains the cytoplasm of metabolically active cells (such as parabasal cells intermediate cells leucocytes and histiocytes as well as cancer cells) a light green colour.
However, the colour balance of a Papanicolaou stained slide depends not only on the staining method used but also the commercial source of the stains and the pH of the cell sample. In consequence the Papanicolaou stain cannot be considered to be an exact stoichiometric stain although the correlation between Pap stained and Feulgen stained material is reportedly very high.For further information about the Papanicolaou stain and general cytopreparatory techniques please consult Chapter 34 , Cytopreparatory Techniques by CM Keebler in Comprehensive Cytopathology (1996) editor M Bibbo pub Saunders
Screening and reporting
- A protocol for screening should be agreed with the laboratory manager and quality control measures and appropriate staff/ workload ratios should be in place.
- In order to develop expertise it is essential that cytotechnologists see a wide range of cases. Laboratories should process at least 15,000 cervical smears per year and each screener may be expected to screen 50 -80 smears per day with breaks at hourly intervals. He / she should have his/her own workstation and binocular microscope.
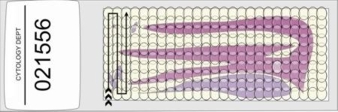
- The prepared slides should be read using a 10x objective and 10x or 12x eye pieces The same objective should be used to screen the whole slide. The screener should progress field by field across the slide until the whole area under the coverslip has been inspected. It is usual to start screening at one corner of the slide and work in a step wise manner until the whole area under the coverslip has been examined as shown below.
- It has been estimated that ,using low power objectives, a screener will have examined 250-300 fields of view during the process of screening a single slide . The average time taken to complete this process is 6-10 minutes (range 5 -20 minutes)
- To mark cells of interest , a 4x objective should be used. A protocol for marking cells should be in place in the laboratory.
- To inspect areas of special interest a 20x or 40x objective should be used.
- The cytology request form and cervical screening history should be provided with each smear and should be reviewed by the cytologist before examining the smear
Terminology and principles of reporting cervical smears
- The cytology report should be accurate and concise so that its contents are easily understood by the smear taker and relevant medical, technical and administrative staff involved in cervical cancer screening.
- The terminology must communicate clinically relevant information to the smear taker. It is generally agreed that this is best achieved if the report is written in free text (narrative) form as shorthand reports are open to misinterpretation. For this reason, numerical classification systems including that proposed by Papanicolaou (1954)are strongly discouraged.
- The type of specimen e.g. cervical scrape, endocervical brush or LBC sample, should be noted.
- There should be a comment on specimen adequacy i.e. whether the specimen is satisfactory for evaluation
- The report should include a brief description of the cytological findings in terms which are widely used and understood
- In cases where the cytological findings are abnormal, the probable pathological changes in the cervix should be predicted.
- A fifth part of the report includes suggestions for management but this is optional.
- The cytologist should take into accounts all the relevant clinical data concerning the patient before preparing the report.
- It is strongly recommended that all abnormal smears are reported by a pathologist or a suitably qualified practitioner who has special training in gynaecological pathology.