Screening error and need for quality assurance
- The Papanicolaou smear test is performed worldwide in order to detect cervical cancer at its earliest stages when treatment is most effective.
- Recent studies have shown that the smear test saves many lives.
- In order for the test to be effective the cervix must be sampled with care, the smear prepared and processed correctly , and the slide analysed and reported by the laboratory with a high degree of accuracy
- This module explains how to achieve and maintain a high standard of performance in the cytology laboratory.
Quality assurance is essential to minimise the risk of errors of cervical cytology
There are 3 main types of errors:
- Errors in smear taking.
- Errors in laboratory processing.
- Errors in interpretation.
Up to 10% of smears from women with pre-invasive or early invasive cancer of the cervix may not contain abnormal cells. There are three possible explanations for this:
Errors of smear taking: Errors of fixation and processing: Errors in the interpretation of the smear 1 The area of the transformation zone was not adequately sampled 1 Cell morphology not clearly displayed 1 Loss of concentration when screening 2 The abnormal cells were not transferred from spatula to slide 2 Nuclear/ cytoplasmic ratios distorted 2 Habituation- A subconscious process by which the brain ignores a repeated signal. 3 The CIN lesion may be small and/or high in the endocervical canal beyond the reach of the spatula 3 Nuclear outline and chromatin structure poorly displayed 3 Distraction from screening (a telephone call) 4 Pale nuclear staining and loss of nuclear/ cytoplasmic contrast 4 Failure to screen the whole slide 5 Clerical error in mislabeling of slide 5 Fatigue (screening too many slides at a sitting) 6 Unsatisfactory microscope optics or laboratory ergonomics 7 Clerical error in recording results Pitfalls of Diagnosis
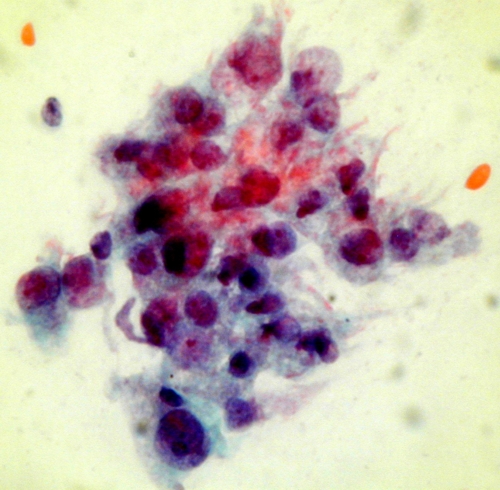
False negative reports are issued when the cytologist fails to detect abnormal cells which are present in the smear .There have been several studies to determine the characteristics of false negative smears. These have shown that the risk of a false negative smear report being issued is very high if the smear contain relatively few ( < 50 ) abnormal cells. Unfortunately studies have also shown that the majority of false negative smear reports are issued in cases where the smears contain a very large number of abnormal cells indeed. In fact , in retrospective studies of women who have presented with invasive cancer it has been found that up to three or more consecutive false negative reports have been issued over a period of several years before invasive cancer was diagnosed . False negative reports are particularly harmful because they result in failure to diagnose or treat the patient's cancer .
False Positive reports are issued when the negative smears are reported to contain abnormal cells . In these cases the patient may undergo unnecessary surgical procedure and may be alarmed unnecessarily.
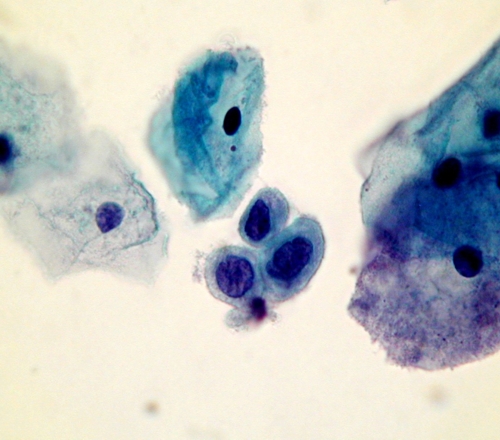
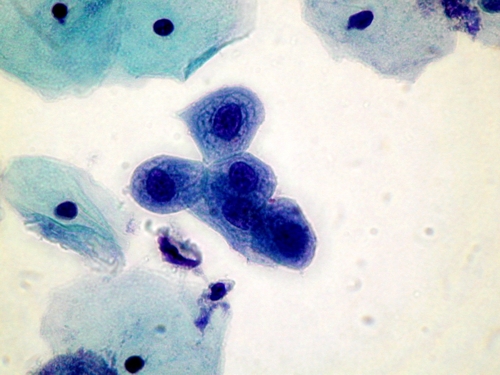
A number of conditions have been recognised which may give rise to problems of interpretation leading to a false positive diagnosis. These so called pitfalls in diagnosis are listed and several are illustrated belowPitfalls in diagnosis
- Small cell dyskariosis
- Follicular lymphocytic cervicitis
- CIN3 minibiopsies ( microbiopsies ) or CIN involving crypts
- Discrete small keratinised cells
- Endometriosis , tubo endometrioid metaplasia
- Lower segment endometrium
- Immature metaplastic cells
- Histiocytes
- radiation changes
NOTE
Up to 45% of smears from women with advanced invasive cancer may not contain abnormal cells . This is because the lesions are often necrotic and ulcerating and the smear is comprised entirely of blood , polymorphs and necrotic debris (the so called malignant diathesis) Cytotechnologists can minimise this problem by systematically evaluating the smears for their adequacy and advising smear takers of the quality of their smears.