| Normal Cell Content |
| Basic Smear Patterns |
| Smear adequacy: The unsatisfactory smear |
Specimen adequacy
- It is important to remember that, like all screening test the cervical smear test is not 100% effective in detecting abnormalities present in the cervix.
- The reasons for failure of the Pap test to contain abnormal cells in the presence of a histologically proven preneoplastic or neoplastic lesion can be attributed to:
- The biological properties of the tumour
- Failure of sampling
- Failure of screening
Biological properties of the tumour: Studies have shown that small CIN lesions (< 0.5cm diameter) and lesions that are high in the endocervical canal are more likely to be missed . Regular smear tests at 3 or 5 yearly intervals will minimise the risk of missing CIN lesion when they are in the earliest stages of development.
Failure of sampling: A neoplastic lesion may be missed if the whole of the transformation zone is not sampled. This can happen if the sampling device selected by the smear taker is not appropriate for the shape or size of the cervix, A neoplastic lesion may also be missed if abnormal cells present on the sampling device are not transferred to the slide. Great care should be taken by the smear taker to ensure that as much material as possible is transferred from spatula to slide. It is anticipated that the use of Liquid based cytology will resolve this problem.
Failure of screening: A false negative report may be issued if the primary screener fails to detect abnormal cells in the smear. This risk can be minimised if appropriate quality control measures are in place in the laboratory (LINK to module on Quality Control).
Evaluation of specimen adequacy by the screener is considered by many to be the single most important Quality Assurance procedure that can be provided by the laboratory
Definition of adequacy
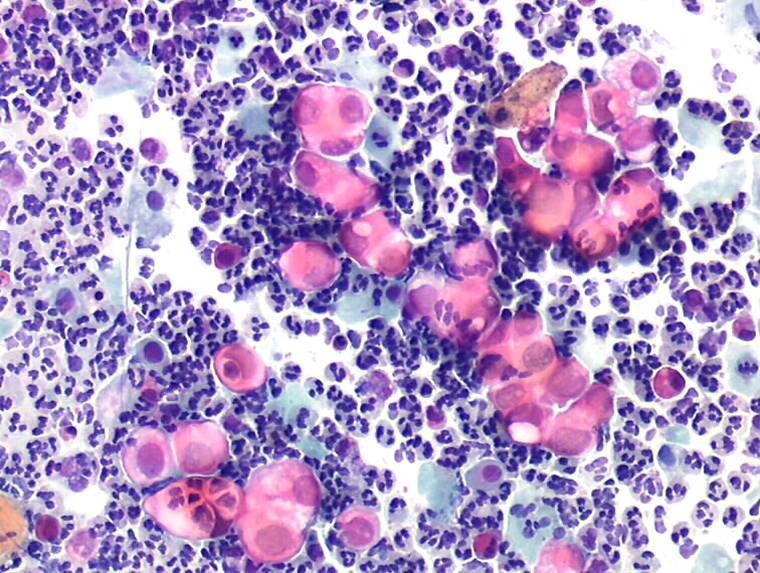
Slides may be unsatisfactory for evaluation for any of the following reasons:
- If the degree of cellularity is insufficient taking into account the woman’s age and hormone status
- If the smear is entirely composed of of separated superficial cells suggesting a vaginal rather than a cervical origin
- If it is poorly fixed or air dried to such a degree that assessment is impossible
- If the cellular material is thickly spread or is obscured by blood , debris , polymorphs ,bacteria or sperm
- If it is entirely composed of endocervical cells unless the only object of the test was to sample the endocervical mucosa.
- A cervical smear or LBC preparation must not be reported as inadequate if abnormal cells are present
- Colposcopy is advisable after three consecutive inadequate smears