

| Detecting HPV |
| HPV DNA testing in clinical practice |
| Immune response |
| Clinical trials and vaccines |
Association of human papillomaviruses and cervical cancer
Human papillomaviruses (HPV) are members of a family of viruses known as the Papovaviruses. They are epitheliotropic viruses which promote cell proliferation which results in the development of benign papillomatous lesions of the genital tract upper respiratory tract, digestive tracts and cutaneous lesions of the skin. More than 70 distinct HPV types have been identified as a result of molecular hybridisation of DNA extracted from condylomata or warty lesions from a variety of sites. Each virus type has a very restricted site of infection and viruses which occupy similar niches appear to be genetically related. Molecular hybridisation of anogenital warts and cervical biopsies have shown that about 30 of the 70 distinct types of HPV are confined to the female genital tract.
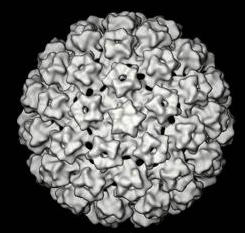 |
| Electronmicrograph of human papillomavirus |
The papillomaviruses are small non enveloped DNA viruses which are approximately
55 nm in diameter. The genome is composed of a double strand of DNA and has three functional coding regions: a region for coding early viral function (E), a region for coding late viral function (L) and a long control region (LCR) which lies between them
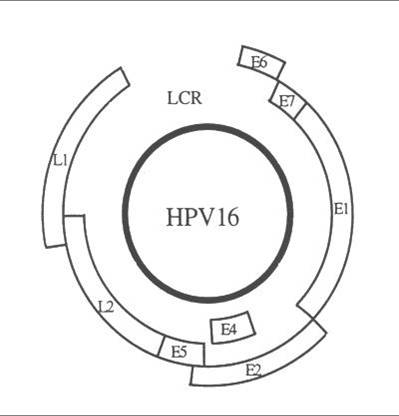 |
| HPV 16 genome structure : the coding regions are represented by open boxes (Phillips AC and Vousden KH 1998) |
| Gene/Region | Function |
E1/E2 |
Code for proteins which control the function of E6 and E7 genes. |
| E4 | Function largely unknown but may control virus release from cell. |
| E5 | Codes for a hydrophobic protein which enhances immortalisation of the cell. |
| E6 | Codes for proteins which inhibit negative regulators of the cell cycle .E6 products inhibit p53 which is a transcription factor for apoptosis (programmed cell death). |
| E7 | Codes for products which bind to the retinoblastoma tumour suppressor proteins thereby permitting the cell to progress through the cell cycle in the absence of normal mitogenic signals. |
| L1/L2 | Code for structural proteins and formation of complete virus particles. |
| LCR | Necessary for normal virus replication and control of gene expression. |
Natural Histology of HPV infection of the cervical epithelium and morphological changes associated with infection
Age at acquisition of HPV infection: HPV is one of the most common sexually transmitted disease.75% of women have acquired at lease one HPV infection by the age of 50. Infection rates are highest in early teens and twenties. After this,rates appear to decline possibly through the development of immunity to the virus. However the virus may persist as a small focus of latent infection which is detectable only at the molecular level.
Route of infection : HPV enters through the basal layer of the epithelium (usually the TZ) where the virus replicates as the basal cells divide. The virus may persist in the basal layer in latent (inactive) form or may continue to replicate as the basal layers differentiate and rise through the epithelium where the cytopathic effect can be recognised in histological section and cytological smears as koilocytosis, parakeratosis and individual cell keratinisation. The acute infection may clear with spontaneous regression of the lesions or may persist as latent infection which may reactivate at any time.
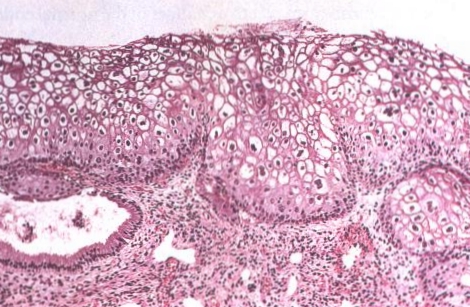 |
| LSIL (CIN 1) of the cervix. This lesion, also known as flat confyloma, is characterized by koilocytotic atypia and usually is associated with HPV 6 or HPV 11 infection. |
Evidence to support concept that HPV have a role in cervical carcinogenesis
In the 1977, two reports were published that suggested for the first time that human papillomaviruses may be present in cervical epithelium (Meisels & Fortin 1977; Purola & Savia 1977). These authors noted the presence of koilocytes in cervical smears and biopsies from women with CIN . They described the lesions in the cervical epithelium which exhibited warty changes which they designated "flat warts "or "non condylomatous warts" . Further research confirmed the presence of wart virus antigen and HPV DNA in the nuclei of the cervical epithelial cells and established that the presence of HPVDNA was often of associated with a focus of CIN 1. Their observations provided the stimulus for extensive research into the role of HPV in the genesis of cervical cancer (Coleman and Richman 1983).
DNA analysis of anogenital warts,CIN and cervical cancerous tissue has shown that two groups of HPV can be identified in the female genital tract. One group of HPV is almost always associated with low grade CIN lesions and exophytic anogenital warts which have a low risk of progressing to cervical cancer . A second group of viruses is found most commonly in CIN2 and CIN3 which have a high risk of developing into invasive cancer.
The oncogenic potential of the viruses in the different groups has been extensively studied and is discussed below.
HPV types found in the female genital tract
| High risk | |
| 16,18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68 may lead to Invasive Cancer | |
| Low risk | |
| 6, 11, 42, 44, 53, 54, 62, 66 may lead to condylomata | |
HPV oncogenic potential
The ability of the viral genes to alter the properties of epithelial cells has been extensively investigated and shows that viruses in the high risk group have remarkable oncogenic properties. In vitro assay of human keratinocytes (which ressemble the normal target cell of the virus) which have been transfected with low risk and high risk HPV types shows that viruses from the high risk group (HPV16 and HPV 18) have the ability to immortalise primary human keratinocytes ie extend their lifespan. In comparison viruses from the low risk group (HPV-6 and HPV -11) do not extend the life span of transfected human cells which mature and die at the same rate as non infected cells. Similarly the low risk viruses perform poorly in experiments concerned with the malignant transformation of rodent cells in comparison to the high risk HPV types. Moreover, HPV-16 and HPV -18 infected human keratinocytes in raft culture (an organotypic culture medium) exhibit a differentiation pattern very similar to that seen in vivo in CIN.
The most convincing evidence that the human papillomaviruses play a key role in the oncogenic process is based on the integration patterns of HPVDNA in cervical cells . Whereas HPVDNA is normally maintained episomally (ie separate from the nucleus) in condylomata and low grade CIN, in cancerous lesions the viral DNA is integrated into the genome of the host cell . Integration results in the disruption of E1 and E2 gene expression which normally control the E6 and E7 genes and deregulation of the E6 and E7 open reading frames. The proteins products of the E6 and E7 coding regions block the normal function of the tumour suppressor genes (p53 and the Rb tumour suppressor gene) thereby allowing cells to bypass normal cell cycle check points at G1 and G2, resulting in unchecked cellular proliferation.
Integration is a consistent finding in all cancers harbouring the high risk virus types HPV16 and HPV18 and provides the strongest evidence that HPV16 and HPV18 play an important role in the development of cervical cancer. HPV DNA is present in 90% of all cervical invasive cancer.
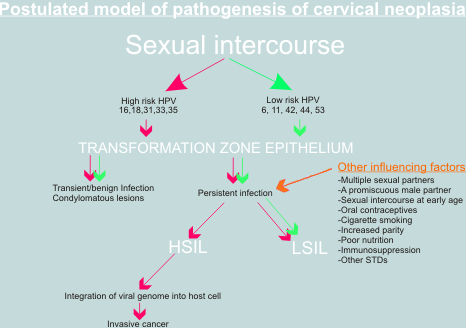 |
Importance of co-factors
It is widely acknowledged that simple infection with high risk HPV or even integration of HPV 16 into the host cell nucleus, is not sufficient for full malignant transformation of the cervical epithelium. Infection of the genital tract with HPV 16 is relatively common whereas invasive cancer is rare; and integration has been detected in some cases of genital warts and CIN lesions. A number of possible Co-factors have been proposed such as impaired immune response, persistence of virus, smoking and administration of steroid hormones (as oral contraceptives). Other genetic events such as loss of tumour suppressor genes and the activation of oncogenes may also play a role . Mutations in ras ,fos and other oncogenes have been detected in cervical cancer cell lines but their role in vivo is still to be determined . Despite the gaps in our knowledge, the papillomaviruses have provided a unique insight into the mechanisms of carcinogenesis and have led to advances in our knowledge of the aetiology of cervical cancer which have implications for the future in terms of prevention (through vaccination), early diagnosis (through HPVDNA screening) and treatment (through antiviral drugs) of this critical disease.
Association of HPV and cervical cancer: relative risk is very strong and is comparable to that of chronic hepatitis B and liver cancer and greater than that of smoking and lung cancer.