| Spleen |
SPLEEN CYTOLOGY
- General
- Classification of splenomegalies
- Anatomy
- Focal and diffuse splenomegalies and Fine needle cytology
- Normal constituents
- White pulp hyperplasia
- Acute, suppurative and necrotizing processes
- Myeloid metaplasia
- Granulomatous processes
- Storage diseases
- non Hodgkin lymphoma
- Hodgkin lymphoma
- Malignant hystiocytosis
- Metastases
General
Nodular or diffuse splemomegalies may be determined by several causes; in most cases, clinical and serological data are diagnostic but there are still rare conditions or equivocal clinical presentations in which a direct evaluation may be useful. FNC of the spleen was first used for the diagnosis of leishmaniasis and was then extensively used in the Seventies by the Swedish school. The fear of haemorrhage first, and the subsequent development of other non invasive diagnostic techniques have limited, over time, the diffusion of splenic FNC. Nonetheless, there still are some clinical situations in which FNC, despite some prejudices, may contribute to the diagnosis of nodular or diffuse splenomegalies. Moreover splenic FNC may be conveniently performed under ultrasound control, without complications, provided that the whole procedure is properly performed, and being aware that mononucleosis and hemorrhagic diathesis are definitive contraindications.
Back to topDisordes associated with splenomegaly
INFECTIONS
- Non specific splenitis
- Mononucleosis
- Tuberculosis
- Brucellosis
- Malaria
- Histoplasmosis
- Leishmaniosi
- Echinococcosis
CONGESTIVE STATES
- Cirrhosis
- Portal or splenic thrombosis
- Cardiac failure
AUTOIMMUNE DISEASES
- Rheumatoid arthritis
- Systemic lupus erythematosus
STORAGE DISEASES
- Gaucher disease
- Niemann-Pick disease
- Mucopolysaccaridoses
LYMPHOHEMATOGENOUS DISORDERS
- Hodgkin’s lymphoma
- Non-Hodgkin lymphomas
- Histiocytoses
- Multiple myeloma
- Myeloproliferative syndromes (CML, polycytemia vera, myelofibrosis, myeloid metaplasia)
- Leukemias
- Hemolitic anemias
MISCELLANEOUS
Back to top Back to top
- Amyloidosis
- Primary neoplasms and cysts
- Metastases
Anatomy
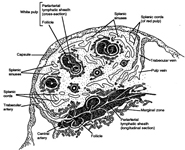
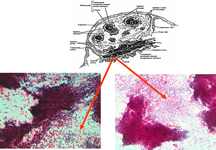
The spleen is the organ that filters blood and is part of the immune system. It is found in the upper left quadrant of the human abdomen, and measures approximately 6 to 16 cm. in length in healthy adults. The organ is contained in a thin capsule from which thin fibrous trabeculae enter into the parenchyma. The spleen is composed of red pulp, which has haemocatheretic functions, and white pulp which has immunologic functions. The red pulp consists of a fine meshwork of fibers in continuity with those of the trabeculae, and contain the cords of Billroth and sinusoids in which the blood flows. The white pulp consists of periarteriolar lymphoid sheets (PALS), which represent the T-zone, and of lymphoid follicles, which represent the B-zone.
Back to topFocal and diffuse splenomegalies
Focal
- Hodgkin’s lymphoma
- Large-cell, non-Hodgkin’s lymphomas
- Primary neoplasms and cysts
- Metastases
- Tuberculosis
Miliary or diffuse
- Infections
- Congestive splenomegaly
- Autoimmune diseses
- Small-cell, non-Hodgkin lymphomas
- Histiocytoses
- Multiple myeloma
- Myeloproliferative syndromes
- Leukemias
- Hemolitic anemias
- Amyloidosis
Fine needle cytology
FNC has to be performed using 23-25 gauge needle; the sub-costal approach is preferable. The patient has to be prepared and invited to hold the breath upon insertion of the needle and during aspiration; a US guide helps reach the target. Few quick movements back and forth may be performed in aspiration. Smears, microscopic evaluation and management of the material have been described above. After the FNC, US control should be performed and the patient should rest in bed, possibly with ice packs on the splenic area for a few hours.
Back to topNormal constituents
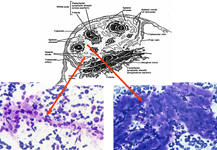
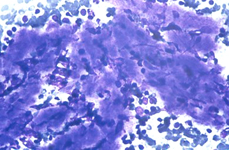
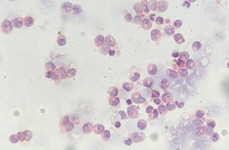
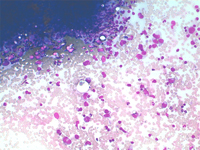
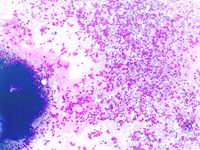
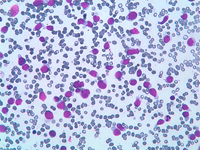
The constituents of the red pulp are generally poorly represented on sFNC, while platelets, macrophages and scattered fibrous or endothelial cells may be observed. The white pulp is represented by dense fragments of tightly packed lymphoid cells, with nuclear details observable only at the edges of the fragments. Invariably one or two vascular structures enter these groups, which represent the periarteriolar lymphoid sheaths (PALS). Other than PALS, scattered lymphoid cells in different stages of maturation may be present on the smear, representing the B-cell component. PALS and scattered lymphoid cells may vary quantitatively at different ages and in different immunologic stages, being numerous in childhood and in heightened immunologic stages.
Red pulp
Red pulp is scantily represented on cytological smears: sinus histiocytes, occasional endothelial cells and groups of platelets.
White pulp
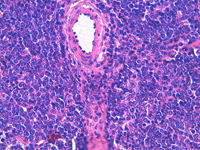
White pulp is mainly represented by dense fragments of lymphoid cells tightly attached to each other with one or two vascular structures entered the groups; nuclei are observable only at the edge of the fragments. These fragments are the cytological counterpart of periarteriolar lymphoid sheaths (PALS) which represent the T zone of splenic white pulp.
White pulp: follicle cells
White pulp is also represented by dispersed lymphoid cells at various grades of maturation, which mainly represent the follicular, B-cells, of white pulp.
Back to topWhite pulp hypeplasia: PALS (T-cells) and follicles (B-cells)
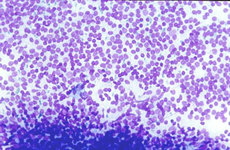
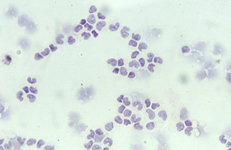
In white pulp hyperplasia smears are highly cellular with numerous and large PALS and dispersed lymphoid cells. White pulp hyperplasia may be observed in infective diseases or immunologic disorders, in children is mainly related to a heightened immunologic state.
Back to topAcute, suppurative necrotizing processes
Abscesses, may occur in blood-borne infections; sometimes along leukaemia or immunodeficiency syndromes. Sometimes these processes cause sub acute inflammatory processes with histiocytes and multinucleated giant cells. Infarcts are more common mainly caused by systemic or infective emboli determining coagulative or suppurative necrosis.
Back to topMyeloid metaplasia
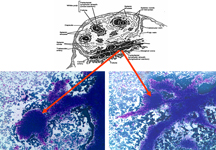
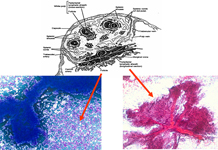
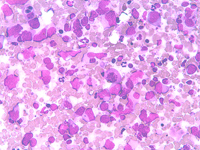
Myeloid metaplasia may occur mainly in cases of myelofibrosis and myeloproliferative syndromes but may be observed in different pathological condictions such as lymphomas, previous infective diseases or after chemotherapies. The hallmark of this condition is the presence of megakaryocytes, which may be isolated or attached to PALS
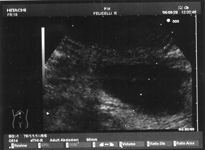
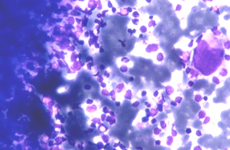
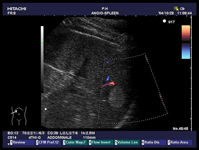
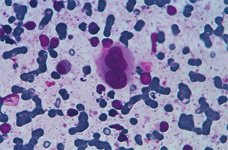
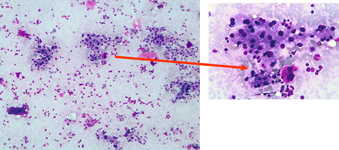
Myeloid metaplasia causing diffuse splenomegaly (note the tip of the needle), corresponding smear shows myeloid cells at different stages of maturation and a megakaricyte. Note the PALS on the left. Myeloid cell may be identified by CD13+ and differentiated from lymphoid cells by HLADR+.
The hallmark of myeloid metaplasia are megakaryocytes, isolated or entrapped in the PALS, mature or immature (upper right), sometimes simulating Reed Sternberg cells or showing bizarre fashions.
Myeloproliferative syndromes and myeloid metaplasia
Myeloid metaplasia may occur along myeloproliferative syndromes such as myelofibrosis or trombocyosis. Note mature and immature myeloid cells and large amount of platelets.
Myeloid metaplasia: liver
Myeloid metaplasia may involve other organs such as the liver. Here in a liver FNA myeloid cells and megakaryocytes are interspersed between hepatocytes.
Back to topGranulomatous processes
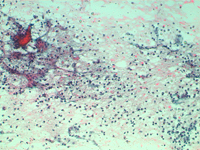
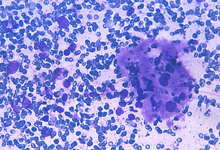
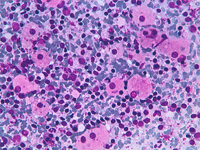
Sarcoidosis and tuberculosis and other infective diseases may cause granulomatous lesions to the spleen. Hodgkin and non Hodgkin lymphoma and chemotherapy may be followed by granulomatous lesions which may cause equivocal clinical features.
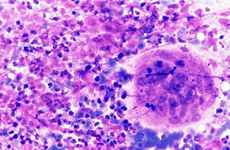
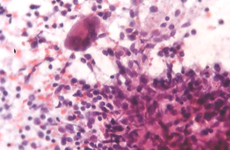
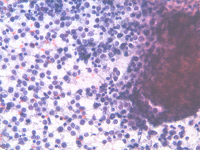
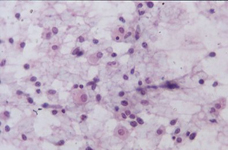
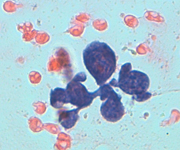
Epithelioid cells (left) and granulomatous structure in a hematic background in a splenomegaly developed in a NHL patient in remission.
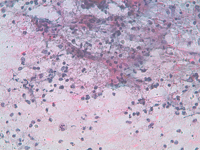
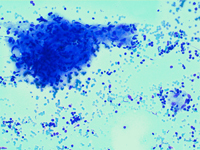
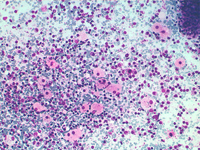
Back to topStorage diseases
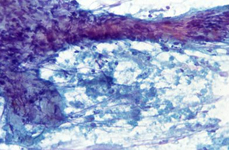
Splenic FNA of storage diseases showing numerous histiocytes with large, pale, bubbly cytoplasm, dispersed or attached to PALS edges. Cytoplasm is typically pinkish or with many small lipidic droplets.
Back to topLymphomas
- Hodgkin (HL) and non Hodgkin lymphomas (NHL) may involve the spleen; HL and large cell NHL may determine nodular lesions as well as primary splenic NHL, which is generally a B- large cell NHL and presents with nodular lesions. Small cell NHL may present as diffuse or “miliaric”. Non invasive diagnostic procedures have drastically reduced the need for direct investigations. Moreover, although primary splenic NHL are generally diagnosed and treated by splenectomy, FNC may be requested in peculiar clinical contexts.
- Cytological criteria and a diagnostic algorithm are almost the same as those described for lymph nodes, whereas the diagnostic criteria for HL relapse are less stringent than for the primary diagnosis.
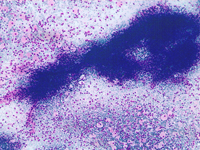
Small cell non Hodgkin lymphoma
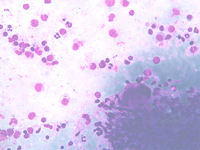
Small lymphocytic lymphoma involving the spleen: a monomorphous cell population of small lymphocytes are interspersed in the background. PALS may be preserved as observed on the top and corresponding histological sample.
Small cell follicular lymphoma involving the spleen: a monomorphous cell population of small lymphocytes are interspersed in the background. PALS are preserved.
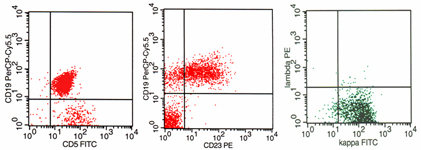
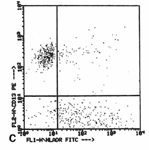
Differential diagnosis between florid white pulp hyperplasia and small cell non Hodgkin lymphoma. FC and ICC evidence of light chain restriction on cytospins (upper right and left) may determine the diagnosis.
Primary lymphoma of the spleen and large cells NHL
Primary NHL of the spleen and large B-cell lymphoma (DLBCL) may generally arise or involve the spleen with single or multiple nodules. Smears show dispersed, atypical, large lymphoid cells. PALS are scantly or not present.
Primary lymphoma of the spleen and large cells NHL showing large atypical, lobulated cells. PALS are scanty or absent.
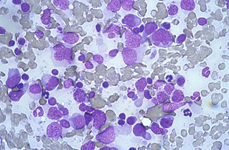
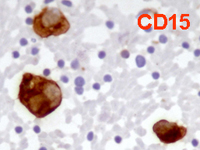
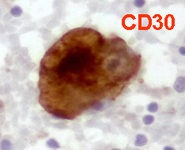
Back to topHodgkin lymphoma
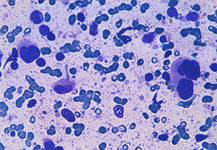
Spleen if frequently involved in advanced stages of HL giving a nodular presentation. Cytological criteria are almost the same of lymph nodes, whereas, in case of relapse, atypical mono and binucleated cells may be sufficient for the diagnosis.
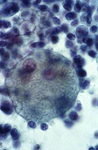
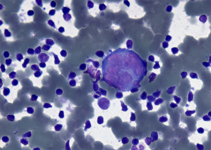
Back to topMalignant hystiocytosis
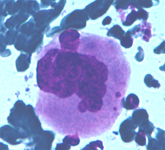
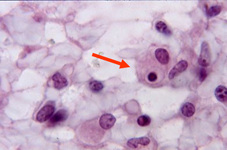
A monotonous proliferation of histiocytes with large, dense cytoplasm and occasional emperipolesis (arrow). Normal constiuent are completely absent. Despite the bland cytological features the disease may be subtle and aggressive.
Back to topMetastases
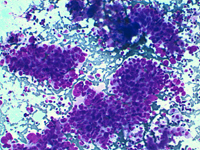
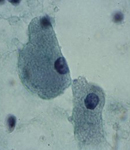
Splenic metastases are extremely rare mainly in the initial phases of the disease. Lung, breast, melanoma and colon tumours are the most frequently reported cases.
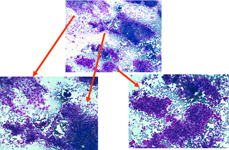
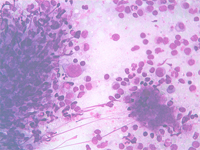
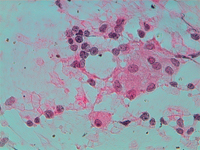
Splenic metastasis from a lung carcinoma; groups of malignant epithelial cells, PALS, few dispersed lymphoid cells and a sheet of benign mesothelial cells transported by the needle.
Back to top